Evaluation of Analytical Chemistry Capability. Accurate results are essential to understanding and solving the problem of arsenic-affected drinking water in Bangladesh; therefore, a major objective of the 1997 field program was to determine the accuracy of analytical chemistry laboratories working on this problem.
The recovery of arsenic from independent standards, sample matrix spikes, and replicate samples was used to evaluate 3 anonymous laboratories that routinely analyze drinking water in Bangladesh (see Table 1). These 3 laboratories would presumably benefit from a more rigorous quality assurance and quality control program, none generated accurate results. The arsenic results from laboratory 1 are systematically high by approximately a factor of 4; therefore, a simple calibration error was likely generating results which incorrectly suggest that the arsenic problem is 4 times worse than reality. Laboratories 2 and 3 only recovered approximately 50% of the arsenic from independent standards, such results underestimate the significance to the arsenic problem by a factor of 2.
| Sample Description | Independent Laboratory |
Result (mg/L) |
|---|---|---|
| Standard solution = 1 mg As/L | Laboratory 1 | 4.891 |
| Distilled water = 0 mg As/L | " | 0.002 |
| Sample A (0.25 mg As/L) | " | 1.101 |
| Sample A (Blind duplicate) | " | 1.035 |
| Sample A (Blind triplicate) | " | 0.266 |
| Sample A plus 6.3 mg As/L | " | 24.126 |
| Sample B (0.31 mg As/L) | " | 1.109 |
| Standard solution = 1 mg As/L | Laboratory 2 | 0.533 |
| Sample B (0.31 mg As/L) | " | 0.397 |
| Sample B plus 3.3 mg As/L | " | 0.884 |
| Standard solution = 1 mg As/L | Laboratory 3 | < 0.5 |
| Sample B (0.31 mg As/L) | " | 0.30 |
| Sample B plus 7.1 mg As/L | " | > 1.0 |
In contrast, the ICDDR,B laboratory performed exceptionally well during the 1997 field program (see Table 2). The recoveries from the blind analysis of all independently prepared standards and sample matrix spikes for arsenic, sulfate, chloride, and phosphate were within the 100 ± 25% range considered acceptable for routine analytical laboratories (12). This is especially impressive because the arsenic analyses were completed without the benefit of an atomic absorption spectrophotometer or similar modern instrumentation. However, the matrix spike recovery from the determination of ferrous iron in undiluted groundwater by the 1,10-phenanthroline method was suppressed by an analytical interference (see Table 2). This interference was defeated by diluting all ferrous iron samples 10 times with distilled water before color development. Similarly, all samples submitted for total iron analysis by the 1,10-phenanthroline method were diluted 10 times with distilled water before acidic digestion to defeat this interference; despite this precaution, 24 of 89 samples (27%) failed to develop proper color or generated a whitish colloidal precipitate upon the addition of 1,10-phenanthroline. The total iron results from these 24 samples were excluded from quantitative evaluation.
| Analyte | Independent Standard Recovery |
Sample Matrix Spike Recovery |
|---|---|---|
| Arsenic (As) | 83% | 89 ± 11% |
| Ferrous iron (Fe2+) | 93 ± 10% | 34 ± 23% without dilution 96 ± 13% with 1 to 10 dilution |
| Total iron (Fe) | 95% | 120 ± 12% with 1 to 10 dilution |
| Sulfate (SO42-) | 106% | 106 ± 20% |
| Chloride (Cl-) | 114% | 90 ± 15% |
| Phosphate (PO43-) | 88% | 84 ± 2% |
An appropriate quality assurance and quality control program should be implemented to determine the accuracy and precision of all analytical results associated with the problem of toxins in Bangladesh’s drinking water. This program should be analogous to that used at ICDDR,B and include the routine and blind analysis of independently prepared standards, sample matrix spikes, field duplicates, laboratory duplicates, field blanks, and laboratory blanks.
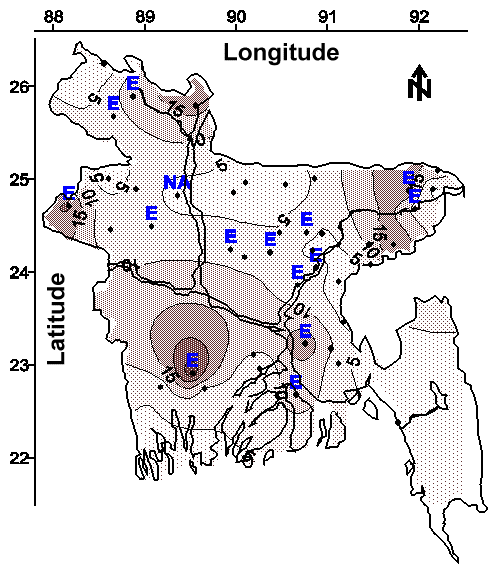
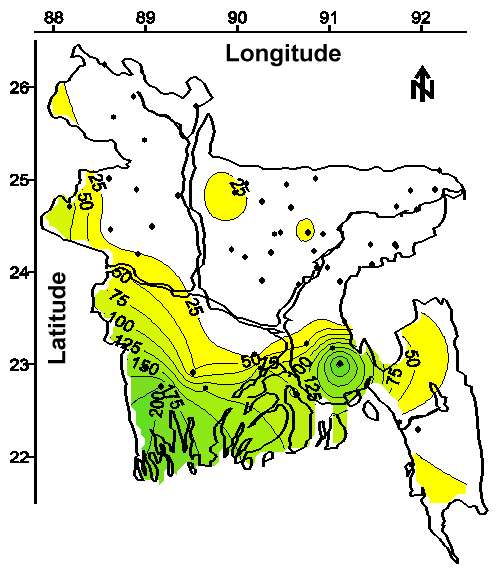
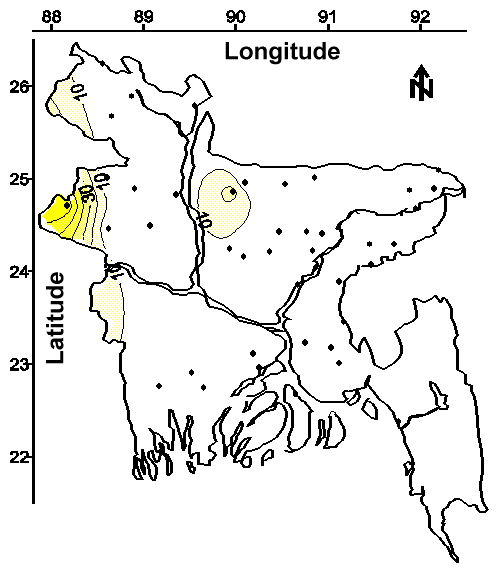
Evidence that Other Potentially Toxic Metals are Widely Distributed in Groundwater. As discussed above, at least 27% of the 2,500,000 tubewells that supply drinking water to this country of 120,000,000 people apparently contain an analytical interference to the 1,10-phenanthroline methods for measuring ferrous iron and total iron. Moreover, the spatial distribution of these tubewells (shown on Figure 2 with the letter "E") suggests that many of the 120,000,000 people in Bangladesh might be ingesting this analytical interference.
 |
This interference could not be further characterized with the laboratory resources that were available in Bangladesh during the 1997 field program; however, the literature suggested that it most likely resulted from one or more potentially toxic non-arsenic metals in tubewell water (8, 13).
Subsequent anecdotal reports of children developing symptoms and dying from chronic arsenic poisoning at exceptionally early ages emerged from Barisal, an area with both arsenic and a relatively high proportion of this interference in tubewell water. A review of the literature suggests the presence of antimony (14), the absence of selenium (15), or the absence of zinc (16) may accelerate the onset of chronic arsenic poisoning.
Therefore, qualitative chemical evidence and anecdotal medical reports suggested that one or more toxic non-arsenic metals may be widely distributed in Bangladesh’s drinking water. As a result of this hypothesis, a second field program was implemented during 1998-1999 to determine the nature, extent, and potential health risks of arsenic and other toxic elements in Bangladesh’s groundwater. The results from our 1998-1999 field program have been published in the peer-reviewed literature and this website.
The Nature and Extent of Arsenic, Chloride, Phosphate, Sulfide, Sulfate, and Total Iron in Groundwater. This evaluation of the nature and extent of arsenic and other inorganic chemicals in groundwater is based on the limited number of samples that could be collected during the 1997 field program. Samples were collected from only 600 of 2,500,000 tubewells in 120 of 68,000 villages; therefore, more extensive studies are required to support or disprove the hypotheses resulting from this field program.
The concentrations of various inorganic chemicals throughout Bangladesh were mapped to identify areas impacted by arsenic, determine the potential source of arsenic, and identify possible water treatment requirements. Contour maps of chemical concentration show the areal extent of arsenic, chloride, phosphate, sulfide, sulfate, and total iron in groundwater (see Figures 2, 3, 4, 7, 8, 9, 10, and 14). These contour maps were drawn using the average chemical concentration in tubewell water from each village, unless otherwise stated (recall that 4 to 6 tubewells per village were sampled). The vertical distribution of arsenic was evaluated by separating samples collected from "shallow" and "deep" tubewells. Shallow tubewells were arbitrarily assigned a depth less than 30.5 m (100 feet) bgs, and deep tubewells were arbitrarily assigned a depth greater than 30.5 m (100 feet) bgs. This separated the samples into two groups with each group containing approximately 300 samples.
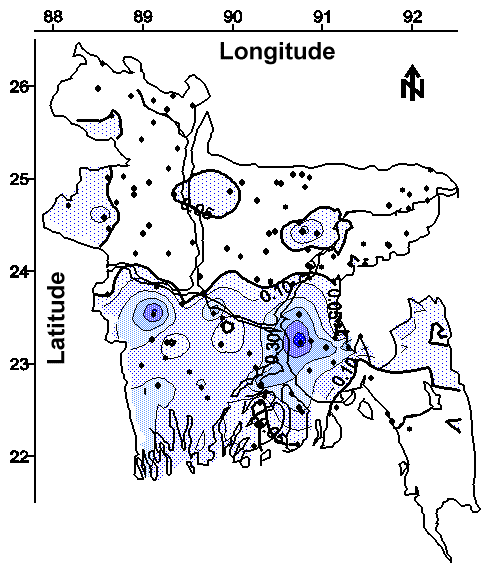
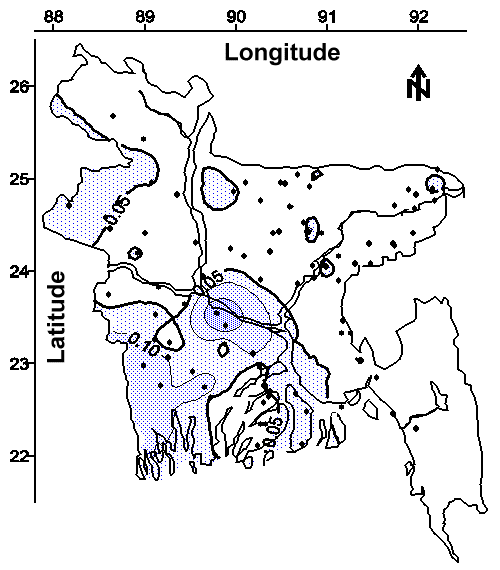
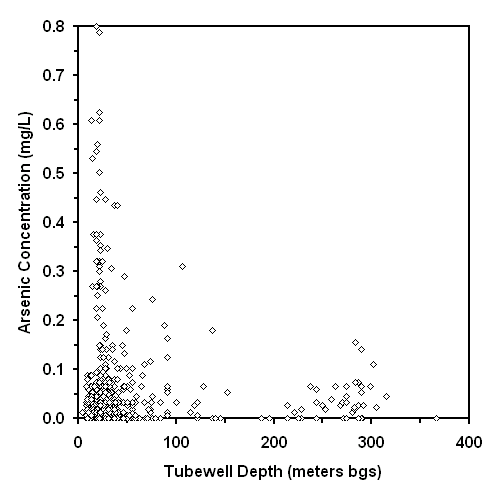
The contour maps of average arsenic concentration in water from shallow and deep tubewells are shown in Figures 3 and 4, respectively. The detection limit for the arsenic method was 0.028 mg/L (defined as the concentration of the most dilute standard used for calibration); therefore, quantitation to the WHO drinking water guideline of 0.01 mg/L was not attempted and these contour maps are based on the Bangladesh drinking water standard of 0.05 mg/L. Approximately 50% of the areal extent of Bangladesh contains groundwater from shallow tubewells with an average arsenic concentration greater than the national drinking water standard (see Figure 3). Approximately 32% of the areal extent of Bangladesh contains groundwater from deep tubewells with an average arsenic concentration greater than the national drinking water standard (see Figure 4). Arsenic was detected in wells ranging from 7.9 m (26 feet) to 315 m (1035 feet) bgs (see Figure 5). The comparison of 10 adjacent pairs (< 120 m or < 393 feet apart) of "very deep" (67.1 to 290 m bgs or 220 to 950 feet bgs) and shallow (< 30.5 m or < 100 feet bgs) tubewells shown in Figure 6 suggests the source of arsenic is hundreds of feet thick in many areas of Bangladesh. This wide-spread areal and vertical distribution suggests the dominant source of the arsenic appears to be one or more geologically deposited minerals.
 |
 |
 |
 |
The distribution of arsenic concentration versus tubewell depth shown in Figure 5 suggests that an arsenic concentration maxima possibly occurs when tubewells are less than 61 m (200 feet) bgs. If this maxima is fact then it may result from younger surface sediments releasing more arsenic than older subsurface deposits, or the preferential release of arsenic into groundwater near the unsaturated zone. If this maxima is fiction then it may result from a statistical aberration produced by the relatively small number of observations (86 of 503 total observations) from tubewells greater than 61 m (200 feet) bgs.
A correlation coefficient matrix for a variety of tubewell water parameters is shown in Table 3 for informational purposes. Interpretation of this data should be performed with caution as it represents the entire country and is probably scale dependent. That is, these correlations may not apply on the district, thana, or village-scale.
| Arsenic | Sulfate | Sulfide | Chloride | Phosphate | Depth | Total Iron | |
| Arsenic | 1.00 | -0.078 | 0.059 | 0.24 | 0.27 | -0.19 | 0.44 |
| Sulfate | 1.00 | 0.41 | 0.051 | -0.060 | -0.14 | 0.16 | |
| Sulfide | 1.00 | 0.062 | 0.19 | -0.097 | 0.23 | ||
| Chloride | 1.00 | 0.10 | 0.028 | 0.38 | |||
| Phosphate | 1.00 | 0.16 | 0.073 | ||||
| Depth | 1.00 | -0.19 | |||||
| Total Iron | 1.00 |
The contour map of average chloride concentration in water from shallow tubewells is shown in Figure 7. The spatial relationship between chloride and arsenic (see Figures 7 and 3) in connate water (water associated with deposition, 17), suggests the arsenic-leaching mineral or minerals were likely deposited in an estuarine environment. An alternative hypothesis is that the release of arsenic from the solid phase is facilitated by chloride or another component of historical seawater; for example, arsenate or arsenite might be displaced from the solid phase into groundwater by anion exchange with chloride.
 |
Phosphate is commonly used as a fertilizer; therefore, the possibility of this agrochemical impacting groundwater must be considered before comparing the spatial relationship between phosphate and geologically deposited arsenic. Phosphate fertilizer forms poorly soluble iron, aluminum, calcium, and magnesium compounds that do not readily leach from most soils (18); however, the phosphate concentration ranged from 1 to 20 mg/L in all 11 groundwater samples collected at greater than 122 m (400 feet) bgs. This range of phosphate concentrations is approximately 5 to 100 times greater than that of seawater (9). Like phosphate, nitrate is associated with agricultural activity. Unlike phosphate, nitrate readily leaches from soils; however, nitrate was measured above the 1 mg of NO3--N/L detection limit in only 5 of 90 groundwater samples. All 5 of the samples with detectable concentrations of nitrate were from wells not greater than 122 m (400 feet) bgs. Four of these 5 samples were from a small area of northwestern Bangladesh (Dinajpur, Phulbari, and Gobandaganj) with relatively sandy soils (19) which often promote leaching (18) and low concentrations of phosphate in groundwater. These findings suggest that the dominant source of phosphate in Bangladesh’s groundwater is geological, not an agricultural leachate.
The contour map of average phosphate concentration in water from shallow tubewells is shown in Figure 8. The spatial relationship between phosphate and arsenic (see Figures 8 and 3) suggests the arsenic-leaching mineral or minerals might contain arsenate isomorphically substituted for phosphate; that is, the major source of arsenic in Bangladesh’s groundwater may be the dissolution of a phosphate or phosphates which have arsenate as an impurity. An alternative hypothesis is that arsenate or arsenite might be displaced from the solid phase into groundwater by anion exchange with phosphate. These hypotheses should be confirmed or disproved by a detailed mineralogical evaluation of a statistically significant number of soil samples.
 |
In contrast, a significantly different hypothesis that arsenic is being released from arsenopyrite (FeAsS) or an iron pyrite (FeS2) has been proposed for the situation in nearby West Bengal, India (immediately west of Bangladesh, 20). This hypothesis suggests that arsenic is initially associated with a poorly soluble pyrite mineral that is underwater in a reducing environment, and the arsenic is released when the pyrite is aerated by lowering the water table during groundwater pumping. This hypothesis is supported by the detailed mineralogical evaluation of an unpublished number of soil samples ("some selected particles from 85 samples...were examined by...X-ray diffraction") from West Bengal which suggested that arsenic is associated with iron pyrite (21).
However, simply finding arsenic in a poorly soluble mineral is not proof that the major source of this element in groundwater has been identified. If the pyrite-source hypothesis is true for the general situation in Bangladesh, then the concentrations of inorganic sulfur and arsenic should be correlated; however, the concentrations of sulfide and arsenic, and sulfate and arsenic in groundwater from Bangladesh are not correlated (see Table 3).
The contour maps of average sulfide concentration and average sulfate concentration in water from shallow tubewells are shown in Figures 9 and 10, respectively. The spatial relationship between sulfide and arsenic (see Figures 9 and 3), and that between sulfate and arsenic (see Figures 10 and 3) suggests the principal arsenic-leaching mineral or minerals in Bangladesh are not associated with inorganic sulfur in contrast to the presumed situation in West Bengal. If the arsenic-leaching mineral or minerals are not associated with inorganic sulfur, then the hypothesis that groundwater depression from irrigated agriculture facilitates the release of arsenic by aerating arsenopyrite or another pyrite might not represent the general situation in Bangladesh. Nevertheless, the occurrence of sulfide and sulfate in groundwater at the West Bengal / Bangladesh border (see Figures 9 and 10) does suggest that pyrites may be an important source of arsenic in West Bengal and limited areas of Bangladesh.
 |
 |
Even if the principal source of dissolved arsenic in West Bengal is pyrites, the general situation in Bangladesh may differ because these two regions have very different geologies. Both West Bengal and Bangladesh receive sediment from the Ganges river basin, but Bangladesh also receives sediment from the Brahmaputra and Meghna Rivers. Each river has different sources of sediment, and most likely different proportions of arsenic, sulfur, and phosphate-bearing minerals. Moreover, Bangladesh is further than West Bengal from the presumed source of pyrites in the Ganges river basin. For these reasons, the percentage of arsenic-containing pyrites in Bangladesh aquifers is likely to differ from, and possibly be much less than that in West Bengal. Furthermore, the environments in which the aquifer sediments were deposited are different in West Bengal and Bangladesh; therefore, the oxidation-reduction conditions, sulfur concentrations, and other important aquifer chemistry parameters are likely different as well. The depositional environments of Bangladesh aquifers commonly include deltaic, estuarine, and riverine sediments, while most of the West Bengal aquifers contain only riverine sediments. Because there are so many substantial differences between the geologies of West Bengal and Bangladesh, it should not be assumed without further study that the source of arsenic in groundwater is the same in both regions.
Various local scientists have used the popular press to advocate restricting irrigation in Bangladesh to prevent the release of arsenic from a presumed pyrite source. However, no experiments evaluating the ability of such a groundwater management scheme to provide safe drinking water have been implemented in Bangladesh, West Bengal, or elsewhere. Moreover, such an unproven groundwater management scheme would have a disastrous effect on the food supply of this famine-prone country. The people of Bangladesh suffered a severe famine in 1974 when its population of 71,000,000 did not have ready access to irrigation and generally relied on 1 crop per year (1). Over two decades later, the 120,000,000 people of Bangladesh are still not food-sufficient despite the 3 to 4 crops per year made possible with irrigation (3). Therefore, any policy that affects the availability of food or water to the people of Bangladesh must be based on meticulous scientific research, not speculation.
The composition of all minerals leaching arsenic into Bangladesh’s groundwater should be determined from a detailed evaluation of a statistically significant number of soil samples. If arsenic is released from arsenopyrite or another pyrite, then alternative groundwater management practices should be evaluated and the treatment of drinking water by aquifer oxygenation may prove to be inappropriate. Aquifer oxygenation is the injection of compressed air or oxygen around a well screen to precipitate arsenic and other metals before they are pumped above ground; however, if pyrites are present, then this process may facilitate the release of arsenic to groundwater. Knowledge of the composition of minerals leaching arsenic into groundwater is relatively unimportant if one is only concerned with treating water after it has been pumped above ground.
The contour map of average total iron concentration in water from shallow tubewells is shown in Figure 2. The spatial relationship between total iron and arsenic (see Figures 2 and 3) suggests the arsenic-leaching mineral or minerals might contain iron. If this arsenic-leaching mineral or minerals also does not contain sulfur (see Figures 9 and 10), then arsenic and iron might be released into groundwater in a reducing environment by dissolution as arsenite and ferrous iron, respectively.
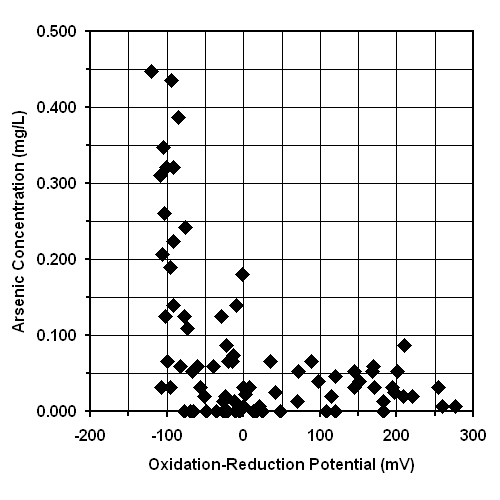
The release of arsenic into groundwater by dissolution in a reducing environment is supported by Figure 11. This graph does not support the hypothesis that arsenic is released from a pyrite source during oxidation. This effect of oxidation-reduction potential on the release of arsenic into Bangladesh’s groundwater is exactly opposite that proposed by advocates of restricting irrigation. Therefore, this untested idea of restricting irrigation might not only start another famine; it could also facilitate the release of arsenic into Bangladesh’s groundwater.
 |
In addition, arsenic and iron can be removed from groundwater in an oxidizing environment by precipitation as arsenate and ferric iron, respectively. The spatial relationship between total iron and arsenic (see Figures 2 and 3), and the general excess of total iron relative to arsenic also suggests that ambient iron might be used to coagulate (coprecipitate) arsenic in a low-input water treatment system (22).
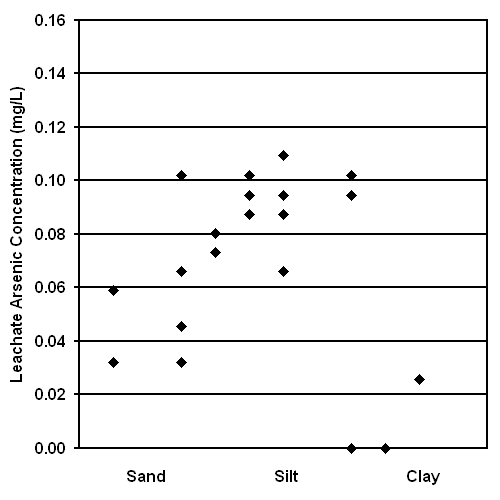
The Leaching of Arsenic from Surface Soils and its Potential Impact on Food Crops. The results of the Soil Leaching Study are shown in Figures 12 and 13. These results suggest that arsenic readily leaches from many Bangladesh surface soils after only 24 hours or 6 days of flooding. Arsenic can be uptaken by crops (18, 23); therefore, this human exposure pathway should be evaluated. Rice is often grown in flooded soils analogous to the conditions of this experiment, is the major staple of Bangladeshi diet, and is grown primarily for domestic consumption (3); therefore, the ingestion of arsenic from domestic rice should be specifically evaluated as a potential human exposure pathway. Unfortunately, the sample preparation equipment required to determine the concentration of arsenic in biological materials is not currently available in Bangladesh.
 |
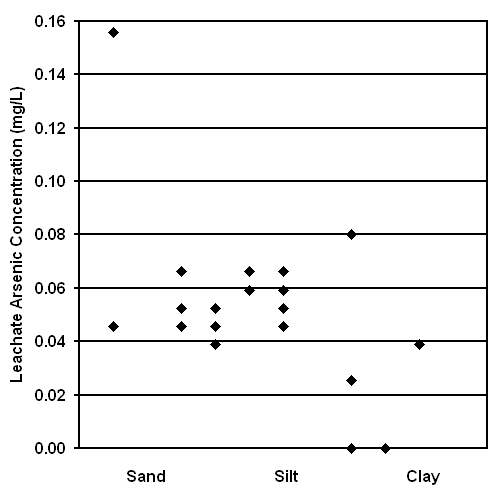 |
The results of this study also suggest that leachate arsenic concentration is greater after 24 hours of flooding than after 6 days of flooding. This result is statistically significant at the 95% confidence level and is based on a paired t-test. This decrease in leachate arsenic concentration over time may result from the precipitation of As(V) after oxygenation by mixing, the precipitation of arsenic by reduced sulfur generated in an anoxic environment, or the precipitation of arsenic by a change in pH (24).
If exposure to arsenic from ingesting domestic food crops is a significant risk to human health, then any factors that decrease leachate arsenic concentration should be evaluated as a way of reducing the uptake of arsenic by these crops. The drinking water standard for arsenic should also be lowered to account for the human health risk associated with ingesting this toxin from food.
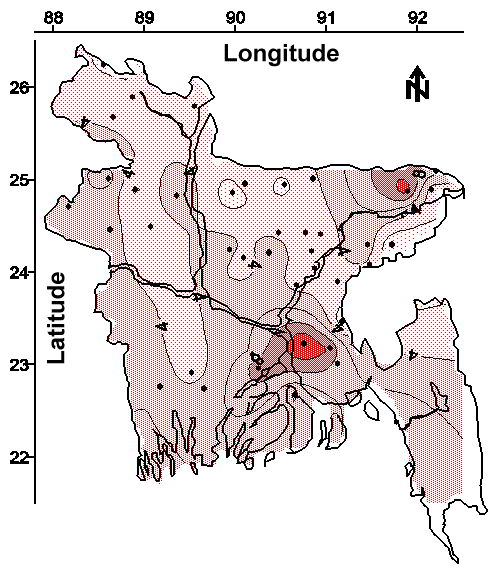
The Potential of Groundwater Monitoring to Reduce the Need for Arsenic Treatment. The contour map of minimum arsenic concentration in water from all (both shallow and deep) tubewells is shown in Figure 14. This contour map was drawn using the lowest ("cleanest") arsenic concentration from the 4 to 6 tubewells sampled in each village. The shaded regions of this map represents areas where no tubewell had an arsenic concentration less than the 0.05 mg/L national drinking water standard. Approximately 15% of the areal extent of Bangladesh contains groundwater with a minimum arsenic concentration greater than the national drinking water standard. This area of Bangladesh will require an alternative drinking water source, or groundwater treatment for arsenic removal. This result also suggests that 85% of the areal extent of Bangladesh has access to groundwater that does not require treatment for arsenic removal prior to drinking.
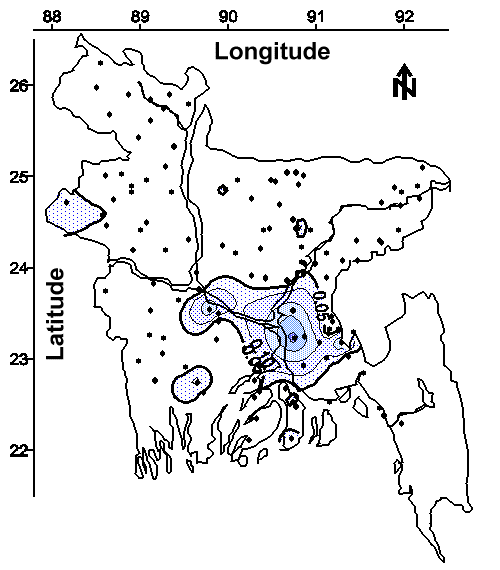 |
Therefore, an intensive groundwater monitoring program identifying suitable drinking water wells within each village would eliminate the need for arsenic treatment for approximately 85% of this 120,000,000 person country. This relatively high success rate coupled with the potential to rapidly complete an initial survey of affected villages at a low cost suggest that groundwater monitoring should be a major component of an overall strategy used to provide safe drinking water to the people of Bangladesh. Once the initial survey is complete, periodic monitoring of selected tubewells should be done to ensure that the population has continued access to safe drinking water. The cost of this program would likely be minimized if the majority of the monitoring was done by trained technicians using specially developed testing kits in the field.
The Potential of Drilling Deeper Tubewells to Reduce the Need for Arsenic Treatment. The Government of Bangladesh and various nongovernmental organizations are currently evaluating a policy of drilling deeper tubewells to access drinking water with arsenic concentrations less than the 0.05 mg/L national standard. Our findings suggest that drilling deeper tubewells will sometimes access "cleaner" water, but will seldom provide ready access to drinking water with arsenic concentrations less than the national standard.
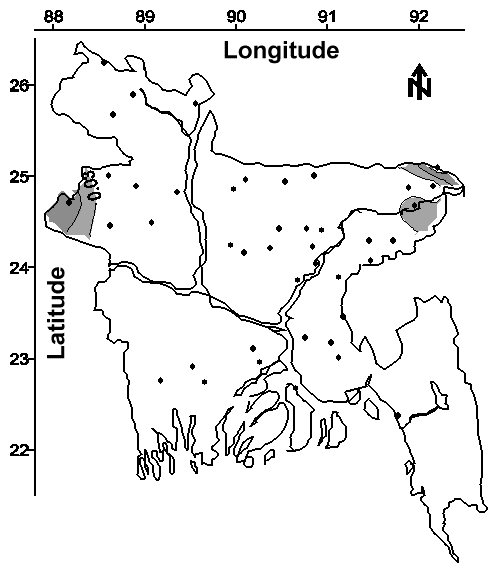
Drilling deeper tubewells will sometimes access groundwater with less arsenic than adjacent shallower tubewells. This benefit is suggested by superimposing Figures 3 and 4, which yields significant areas with higher average arsenic concentrations in shallow tubewells and lower average arsenic concentrations in deep tubewells. Moreover, the negative correlation (r = -0.19) between arsenic concentration and tubewell depth shown in Table 3 suggests that arsenic concentration may decrease with depth in some areas of Bangladesh. Exceptions to this slight trend are noteworthy. The superimposition of Figures 3 and 4 also suggests that drilling shallower tubewells may access water with less arsenic in a relatively small portion of northwestern Bangladesh and other localized areas.
Nevertheless, the success of drilling deeper tubewells will likely be limited because the drinking water it provides will often have arsenic concentrations greater than the national standard. This limitation is suggested by superimposing Figures 3 and 4, which yields significant areas with average arsenic concentrations above 0.05 mg/L in both shallow and deep tubewells. Moreover, this limitation is also suggested by the repeated arsenic concentrations greater than 0.05 mg/L at depths more than 250 m (820 feet) bgs (see Figure 5).
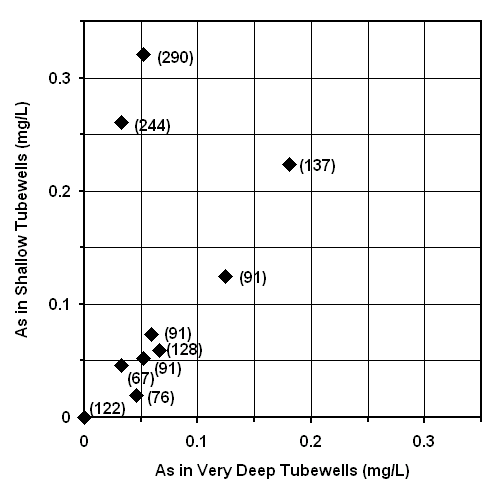
The success rate of drilling deeper tubewells was estimated using Figure 6. These results suggest that drilling deeper tubewells provided access to water with markedly less arsenic in only 2 of 10 cases; however, this frequency may increase by installing even deeper tubewells. The 2 "very deep" tubewells which yielded this improvement were significantly deeper (244 and 290 meters bgs, or 800 and 950 feet bgs) than the remaining 8 "very deep" (67 and 137 meters bgs, or 220 to 450 feet bgs) tubewells. Only 1 of these 2 "very deep" tubewells successfully accessed drinking water with arsenic concentrations less than the 0.05 mg/L national standard. A more extensive study of arsenic concentrations in adjacent "shallow" and "very deep" tubewells should be done to better estimate the success rate of drilling deeper tubewells to access safe drinking water.
Nevertheless, this relatively low apparent success rate coupled with the potential of a prolonged effort at a high cost suggest that drilling deeper tubewells should be a minor component of an overall strategy used to provide safe drinking water to the people of Bangladesh. This approach should likely be restricted to the estimated 15% of Bangladesh that cannot find drinking water with arsenic concentrations less than the 0.05 mg/L national standard from existing tubewells within their villages. Therefore, drilling deeper tubewells will likely complement groundwater treatment to provide safe drinking water for this 15% of Bangladesh.
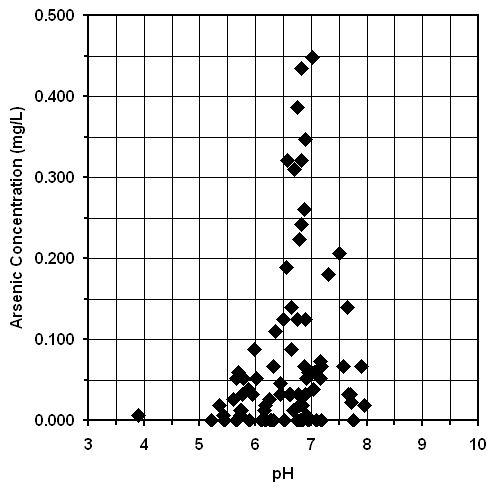
Implications of the Expected Nature of Arsenic in Groundwater on Treatment. The graphs of arsenic concentration versus oxidation-reduction potential and arsenic concentration versus pH are shown in Figures 11 and 15, respectively. The increase in arsenic concentration at relatively low oxidation-reduction potentials and moderate pH values suggests that soluble As(III), rather than poorly soluble and potentially colloidal As(V), was the dominate form in the most highly affected tubewells (24). If the arsenic is in the soluble As(III) oxidation state, then oxidation to poorly soluble As(V) followed by coagulation, filtration, or sorption is required for effective treatment (22).
 |
The Bench-Scale Treatability Study for Arsenic Removal from Groundwater. The effect of chlorinated lime (a locally available oxidant) and ferric chloride hexahydrate (an effective coagulant) on the removal of arsenic from groundwater was evaluated at the bench-scale (see Table 4). The tubewell water used in this experiment was fortified to 2.0 mg of As(III)/L, approximately 3 times the total arsenic concentration of the most severely impacted tubewell found during this project. The results shown in Table 4 suggest that oxidation followed by coagulation can reduce relatively large arsenic concentrations in tubewell water to below the more stringent WHO drinking guideline of 0.01 mg/L. The addition of water treatment chemicals can yield pH values outside of the 5.5 to 8.5 drinking water range recommended by WHO (25); therefore, pH adjustment may be required after coagulation. Limestone (CaCO3) offers inexpensive and effective pH control after coagulation, often without the need of expensive dosing equipment.
| Oxidant (mg/L) |
Coagulant (mg/L) |
Final arsenic concentration (mg/L) |
|---|---|---|
| 4 6 10 15 20 |
250 250 250 250 250 |
0.075 0.037 0.006a 0.003a < 0.002a |
| 20 20 20 20 |
100 150 200 250 |
0.06 0.03 0.02a < 0.002a |
The Conceptual Design of a Pilot-Scale Treatment System for Removing Arsenic and Other Potentially Toxic Metals from Groundwater. The ideal water treatment system for the economic and demographic situation in Bangladesh will effectively remove arsenic and other toxic elements, be inexpensive to build and operate, and be simple to use. Such a system might use atmospheric oxygen as the oxidant and ambient iron as the coagulant; therefore, the long-term expense of purchasing water treatment chemicals would be avoided. Atmospheric oxygen delivered by waterfall or bubble aeration has been routinely used to oxidize As(III) to As(V) in water treatment systems, and should be evaluated in future studies (22). Ambient iron without the addition of another coagulant might adequately separate precipitated arsenic and other toxic metals from water in a large settling tank or an inclined-plate clarifier. Excellent guidance for the construction of low-input water treatment systems for the developing world is provided by the International Reference Centre for Community Water Supply and Sanitation (26) and Heber (27).
The apparent reduction in arsenic concentration from 0.16 to < 0.002 mg/L shown in Table 5 supports the hypothesis that aeration followed by settling without the addition of coagulant can remove arsenic in tubewell water to below the more stringent WHO drinking guideline of 0.01 mg/L. The apparent increase in oxidation-reduction potential suggests the water was aerated when pumped into the storage tank. The apparent decrease in pH, conductivity, and arsenic concentration suggests that ambient iron was oxidized, hydrolyzed water, and precipitated as a ferric hydroxide coagulant of arsenic. The water pump supplying this large tank does not operate at night due to the diversion of electricity to the capital; therefore, coagulated arsenic has many hours of relatively turbulent-free water to settle each evening.
| Parameter | Influent | Effluent |
|---|---|---|
| Arsenic (mg/L) Oxidation-reduction potential (millivolts) pH Conductivity (microsemans) Temperature (°C) Total iron (mg/L) Sulfate (mg/L) Sulfide (mg/L) Chloride (mg/L) Phosphate (mg/L) |
0.16 -38 7.06 514 27.2 NA b NA NA NA NA |
< 0.002a 19 6.47 344 28.2 1.4 < 1 < 0.03 16 1.3 |