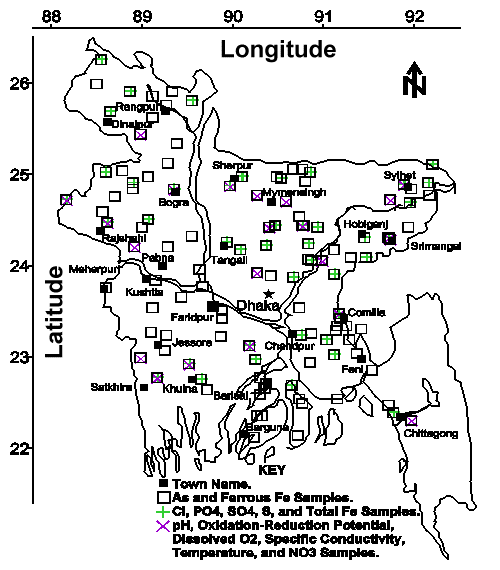
Groundwater Sample Collection, Preservation, Storage, and Analyses. Groundwater samples were collected from approximately 120 villages throughout Bangladesh during July 22 to August 14, 1997. These villages were distributed as evenly throughout the country as practicable to map the distribution of groundwater results on a national-scale (see Figure 1). Typically 4 to 6 tubewells per village were sampled to evaluate the variability of groundwater results on a village-scale.
 |
All groundwater samples were collected, preserved, and stored using a protocol developed by the authors of this study, World Health Organization (WHO), World Bank, United States Agency for International Development (USAID), Government of Bangladesh Department of Public Health Engineering, Geological Survey of Bangladesh, and the Rural Electrification Board of Bangladesh (7). An important component of this protocol was to definitively record the location of each sampled tubewell. Accordingly, the latitude and longitude of all sampled tubewells were determined using the Global Positioning System and large-scale maps. The district, thana, and village of all sampled tubewells were documented. Detailed sketches showing the locations of all sampled tubewells within each village were drawn. The owner and the owner’s reported depth of each sampled tubewell were recorded.
Another important component of this protocol was to ensure that each tubewell sample was representative of groundwater quality by using established collection, preservation, and storage methodologies (8, 9). Accordingly, all sampled tubewells we purged by pumping rigorously for 10 minutes immediately before sample collection. All samples were collected directly into polyethylene bottles. These samples were not filtered. All samples submitted for total arsenic and ferrous iron analyses were acidified to pH < 2 with 10% (volume:volume) HCl and stored in ice-packed coolers immediately after collection. All samples submitted for chloride, phosphate, sulfide, sulfate, and total iron analyses were stored in ice-packed coolers immediately after collection. The temperature of all stored samples was maintained at 0°C to 4°C until immediately before analysis.
All analyses were performed at the Environmental Health Programme laboratory of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), unless otherwise stated. All groundwater samples were analyzed within the Maximum Recommended Storage Time specified in Standard Methods for the Examination of Water and Wastewater (8). Approximately 600 samples were analyzed for total arsenic by the silver diethyldithiocarbamate method (10) and ferrous iron by 1,10-phenanthroline (10, see Figure 1). Approximately 100 samples were analyzed for chloride by mercuric thiocyanate (11), total acid-hydrolyzable phosphate by amino acid (11), sulfide by methylene blue (11), sulfate by barium turbidity (11), and total iron after acidic digestion by 1,10-phenanthroline (11). Approximately 75 samples were analyzed immediately after collection for pH by glass electrode or pH paper (8), oxidation-reduction potential by electrode (8), dissolved oxygen by membrane electrode (8), specific conductivity by electrode (8), temperature by thermocouple, and nitrate by cadmium reduction (11).
The performance of the sampling teams and the ICDDR,B laboratory staff was evaluated by the blind analysis of independently prepared standard solutions, the recovery of known additions of standard solution to samples (sample matrix spikes), the blind analysis of distilled water blanks prepared in the field, the blind analysis of duplicate samples collected by the same sampling team, and blind collection of duplicate samples by different sampling teams. The performance of 3 other local laboratories researching arsenic in Bangladesh was evaluated by the blind analysis of independently prepared standard solutions, the blind analysis of sample matrix spikes, and the blind analysis of duplicate samples.
Soil Leaching Study. A total of 25 surface soil samples (0.3 to 1.2 m or 1 to 4 feet below ground surface, bgs) were collected throughout Bangladesh from July 22 to August 6, 1997. These samples were stored in ice-packed coolers from the moment of collection until the samples were processed at the ICDDR,B laboratory between 3 and 5 days later. Each soil sample was homogenized and analyzed for moisture content by evaporation to a constant mass at 105°C. Following desiccation a mass of field-moist soil equivalent to 100 grams of oven-dried soil was delivered to a clean sample jar, distilled water was added to the jar to make the final mass of water equal to 200 grams, and the contents of the jar were mixed for 5 minutes. For example, if a 12 gram sample of field-moist soil weighed 10 grams after drying, then 120 grams of field-moist soil (this initial condition is equivalent to 100 grams of oven-dried soil and 20 grams of water) was delivered to a clean sample jar with 180 grams of distilled water (this final condition is equivalent to 100 grams of oven-dried soil and 200 grams of water; however, the composition of the soil being leached was never altered by oven-drying). After 24 hours a 50 mL aliquot of water was collected, filtered through a standard glass fiber filter used to remove total suspended solids (8), and submitted for total arsenic analysis. Then a 50 mL aliquot of distilled water was added to the soil/water slurry, and the contents of the jar were mixed for 5 minutes. After a total of 6 days another 50 mL aliquot of water was collected, filtered through a standard glass fiber filter used to remove total suspended solids, and submitted for total arsenic analysis.
Bench-Scale Treatability Study for Arsenic Removal from Groundwater. Tubewell water was collected and used immediately for this treatability study without the addition of sample preservatives. The tubewell water was fortified with arsenic to yield 2.0 mg of As(III)/L. One liter aliquots of fortified tubewell water were delivered to 1 L borosilicate glass beakers. Chlorinated lime (a locally available oxidant, aCa(OCl)2·bCaCl2·cCa(OH)2·dH2O) was added at rates of 4, 6, 10, 15, and 20 mg/L. The solutions were mixed for 1 minute. Ferric chloride hexahydrate (an effective coagulant, FeCl3·6H2O) was added at rates of 100, 150, 200, and 250 mg/L. Each solution was mixed for 1 minute, allowed to settle for 24 hours, then the water column above the precipitate was analyzed for total arsenic.