|
Seth H. Frisbie, Ph.D. / Norwich University and Better Life Laboratories, Inc. Erika J. Mitchell, Ph.D. / Norwich University and Better Life Laboratories, Inc. Thomas Bacquart, Ph.D. / Université de Bordeaux 1 Richard Ortega, Ph.D. / Université de Bordeaux 1 Ahmad Zaki Yusuf, M.S. / Bangladesh Association for Needy Peoples Improvement Mohammad Yusuf Siddiq, Ph.D. / Bangladesh Association for Needy Peoples Improvement Bibudhendra Sarkar, Ph.D. / University of Toronto and The Hospital for Sick Children Presented at the Fifth International Conference on Metals and Genetics in Kobe, Japan, 2011. |
 |
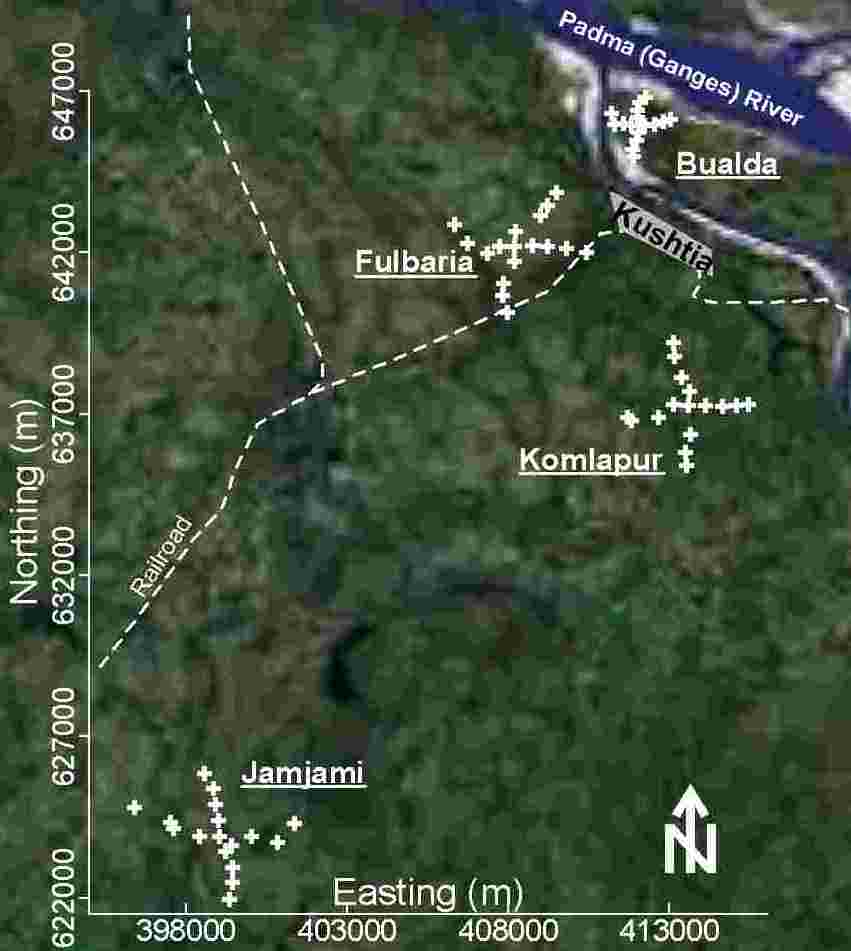
Groundwater samples were collected from 4 neighborhoods in western Bangladesh. These samples were analyzed for every toxic element that has ever been found above World Health Organization (WHO) guidelines in Bangladesh’s drinking water: As, B, Ba, Cr, Mn, Mo, Ni, Pb, Sb, and U. In this study, As, Cr, Mn, Ni, Pb, Sb, and U were found above WHO drinking water guidelines. These results were used to formulate a representative influent, called synthetic tubewell water in this study, for evaluating treatment options under laboratory conditions. The effect of safe, simple, and affordable materials (wood ashes, agricultural calcic limestone, agricultural dolomitic limestone, powdered brick, and iron(III) chloride) on As removal and pH were evaluated using this synthetic tubewell water. Effective treatment to remove As requires the oxidation of soluble As(III) to insoluble As(V), the coagulation of insoluble As(V), the decolorization of Fe(III), the control of pH, and the proper disposal of sludge. Effective treatment to remove the other toxic elements will likely cause even more problems. Therefore, home-scale coagulation for removing As and other toxic elements from western Bangladesh’s drinking water is most likely too problematic for effective use.
 |
Established collection, preservation, and storage methodologies were used to ensure that each sample was representative of groundwater quality (APHA et al. 2005; Stumm and Morgan 1981). Accordingly, all sampled tubewells were purged by pumping vigorously for 10 minutes (min) immediately before sample collection. All samples were collected directly into polyethylene bottles. These samples were not filtered. Samples were analyzed immediately after collection with pH paper, preserved by acidification to pH <2 with 5.0 Molar (M) hydrochloric acid (HCl; BDH Laboratory Supplies, product number 101256J, Poole, England), and stored in ice-packed coolers. The temperature of all stored samples was maintained at 0° to 4° Celsius (C) until immediately before analysis at laboratories in Dubai, France, and Vermont.
These samples were shipped to Dubai and analyzed for arsenic (As) by the arsenomolybdate method (Frisbie et al. 2005). After which, these samples were shipped to France and analyzed for barium (Ba), chromium (Cr), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), and uranium (U) by inductively coupled plasma mass spectrometry (ICPMS; APHA et al. 2005). Finally, these samples were shipped to Vermont and analyzed for boron (B) by the azomethine H method (LaMotte Company 2005), iron (Fe) by flame atomic absorption spectroscopy (FAAS; APHA et al. 2005), and antimony (Sb) by graphite furnace atomic absorption spectroscopy (GFAAS; APHA et al. 2005).
The effects of wood ashes, agricultural calcic limestone, agricultural dolomitic limestone, powdered brick, and iron(III) chloride (FeCl3) on As removal and pH were evaluated using synthetic tubewell water. These ashes were a combustion byproduct from mixed hardwoods harvested in Vermont. They were sieved to >30 mesh prior to use. The calcic limestone (product name Cal-Carb) was purchased from The Old Mill, Inc., North Troy, VT, USA. The dolomitic limestone (product name Pulverized Limestone) was purchased from the Southern States Cooperative, Inc., Richmond, VA, USA. Three grades of bricks were purchased from 6 brickyards in western Bangladesh. These 3 grades of brick were pora int (often black or brown, misshapen by excessive heat in the kiln, used to build roads), ek nombor it (deep red, not misshapen, used to build houses), and dui nombor it (yellowish red, not misshapen, used to build walls). These 6 brickyards were owned by Abdul Hye of Chorhush, Naosher Ali of Boriya, Abdul Sobhan of Baburhut, Abdul Hussain of West Mozompur, Nawab Ali of Nawa Para, and Mamun and Shihab of Nalkupa, respectively. Prior to use, these 18 bricks were crushed, sieved to >50 mesh, and combined to make 1 composite sample. The FeCl3 (product number I89) was purchased from Fisher Scientific International, Inc., Hampton, NH, USA.
The effects of these ashes, limestones, powdered brick, and FeCl3 on As removal and pH were evaluated during jar tests. A 6-position gang stirrer was used for all jar tests (Phipps & Bird, Inc., model number 7790-400, Richmond, VA, USA). Six 1-L beakers were used as jars. Each beaker received 1 L of synthetic tubewell water. The first beaker was a control. The second received 1 g of wood ashes or 1 g of agricultural limestone. The third received 1 g of >50 mesh brick. The fourth received 50 milligrams (mg) of FeCl3. The fifth received 1 g of wood ashes or 1 g of agricultural limestone immediately followed by 1 g of >50 mesh brick. And the sixth received 1 g of wood ashes or 1 g of agricultural limestone immediately followed by 50 mg of FeCl3. A second set of these 6 solutions was prepared as a duplicate. All solutions were stirred for 30 min at 120 revolutions per min. After which, all solutions were allowed to settle for 30 min. The pH of each final solution was measured by glass electrode (APHA et al. 2005). Finally, a sample of each solution was passed through a 0.45-micron filter, preserved by acidification to pH <2 with concentrated nitric acid (HNO3; Spectrum Chemicals & Laboratory Products, product number N1079, Gardena, CA, USA), and measured for dissolved As by GFAAS (APHA et al. 2005; Frisbie et al. 2005).
| Element | Average Concentration (μg/L) |
WHO Guideline (μg/L) |
% of Unsafe Tubewells |
| As B Ba Cr Fe Mn Mo Ni Pb Sb U |
29 19 140 4.7 2,700 800 1.4 11 0.52 1.6 2.5 |
10 500 700 50 NAa 500 70 20 10 5 2 |
33 0 0 1 NA 75 0 3 1 3 48 |
a The WHO has not established a drinking water guideline for Fe (WHO 1996; WHO 1998).
| As | B | Ba | Cr | Fe | Mn | Mo | Ni | Pb | Sb | U | |
| As | 1.00a | ||||||||||
| B | 0.81a | 1.00a | |||||||||
| Ba | 0.26b | 0.40a | 1.00a | ||||||||
| Cr | 0.82a | 0.92a | 0.30b | 1.00a | |||||||
| Fe | 0.82a | 0.92a | 0.40a | 0.97a | 1.00a | ||||||
| Mn | 0.46a | 0.31b | 0.19 | 0.26b | 0.21 | 1.00a | |||||
| Mo | 0.28b | 0.05 | 0.16 | -0.03 | -0.01 | 0.28b | 1.00a | ||||
| Ni | 0.13 | 0.07 | 0.07 | 0.10 | 0.09 | -0.09 | -0.05 | 1.00a | |||
| Pb | 0.83a | 0.94a | 0.33a | 0.98a | 0.96a | 0.28b | -0.02 | 0.09 | 1.00a | ||
| Sb | 0.34a | 0.33a | 0.56a | 0.24 | 0.31b | 0.38a | 0.29b | 0.40a | 0.28b | 1.00a | |
| U | -0.02 | 0.07 | -0.27b | 0.04 | -0.05 | 0.18 | -0.21 | -0.02 | 0.08 | 0.04 | 1.00a |
| Element | Concentration (μg/L) |
WHO Guideline (μg/L) |
% of Unsafe Tubewells |
| As Cr Fe a Mn Ni Pb Sb U |
84 9.5 7,300 870 31 1.2 2.3 0.93 |
10 50 NA 500 20 10 5 2 |
100 5 NA 59 9 5 9 14 |
a Synthetic tubewell water was prepared with ambient iron Fe2+ (7,300 μg/L of Fe2+) and without ambient iron Fe2+ (0 μg/L of Fe2+). The WHO has not established a drinking water guideline for Fe (WHO 1996; WHO 1998).
In addition, ferrous iron (Fe2+) at natural concentrations in Bangladesh’s tubewell water can significantly increase As removal by oxidation and coagulation. That is, the oxidation of soluble As(III) and soluble Fe(II) by dissolved oxygen (O2), chlorinated lime (a locally available disinfectant; aCa(OCl)2•bCaCl2•cCa(OH)2•dH2O), or some other oxidizing agent yields insoluble As(V) and insoluble Fe(III) that coprecipitate and settle out of solution (Frisbie et al. 1999; USAID 1997). In this study the ambient concentration of Fe2+ is 7,300 μg/L, the average Fe concentration from the subset of samples with As concentrations greater than 10 μg/L (Table 3). The effect of ambient Fe2+ on As removal was evaluated by using synthetic tubewell water with 7,300 μg/L of Fe2+ and 0 μg/L of Fe2+ during laboratory testing (Table 4).
| Treatment | Synthetic Water with Ambient Fe2+ | Synthetic Water without Ambient Fe2+ | ||
| % As Removal | Final pH | % As Removal | Final pH | |
| Control | 6.6 ± 6.2 | 5.6 ± 0.1 c | 0.0 ± 0.1 | 5.6 ± 0.2 c |
| 1,000 mg Brick | 19.3 ± 3.7 | 5.9 ± 0.4 c | 0.0 ± 0.0 | 6.5 ± 0.9 c |
| 50 mg FeCl3 | 29.0 ± 3.5 | 3.3 ± 0.1 | 30.9 ± 6.8 | 2.9 ± 0.1 |
| 1,000 mg Wood Ashes | 64.3 ± 0.4 a | 10.2 ± 0.5 | 11.5 ± 0.6 | 10.2 ± 0.1 |
| 1,000 mg Wood Ashes + 1,000 mg Brick | 65.9 ± 1.0 a | 10.5 ± 0.2 | 11.9 ± 0.2 | 10.3 ± 0.1 |
| 1,000 mg Wood Ashes + 50 mg FeCl3 | 96.7 ± 0.6 a, b | 9.8 ± 0.0 | 89.9 ± 0.7 a, b | 9.6 ± 0.1 |
| 1,000 mg Calcic Limestone d | 68.2 ± 2.8 a | 6.5 ± 0.0 c | 0.6 ± 0.7 | 6.8 ± 0.1 c |
| 1,000 mg Calcic Limestone d + 1,000 mg Brick | 70.3 ± 0.8 a | 6.6 ± 0.0 c | 1.6 ± 0.4 | 6.9 ± 1.0 c |
| 1,000 mg Calcic Limestone d + 50 mg FeCl3 | 16.3 ± 0.2 | 4.9 ± 0.3 | 13.9 ± 1.5 | 4.6 ± 0.0 |
| 1,000 mg Dolomitic Limestone e | 53.5 ± 8.5 a | 5.6 ± 0.1 c | 1.3 ± 1.0 | 5.6 ± 0.0 c |
| 1,000 mg Dolomitic Limestone e + 1,000 mg Brick | 50.8 ± 3.2 a | 5.4 ± 0.2 | 1.2 ± 0.5 | 5.6 ± 0.1 c |
| 1,000 mg Dolomitic Limestone e + 50 mg FeCl3 | 17.7 ± 1.7 | 4.7 ± 0.1 | 17.2 ± 0.0 | 4.2 ± 0.4 |
a Meets the 50 μg/L Bangladesh drinking water standard for As on average.
b Meets the 10 μg/L WHO drinking water guideline for As on average.
c Meets the 5.5 to 8.5 WHO drinking water guideline for pH on average (WHO 1984).
d Calcic limestone was 95.5 ± 0.7 % CaCO3 and 5.0 ± 0.5 % MgCO3.
e Dolomitic limestone was 55.3 ± 0.1 % CaCO3 and 45.0 ± 0.5 % MgCO3.
In contrast, ambient Fe2+ did not significantly increase As removal if 50 mg/L of FeCl3 was added as a coagulant, according to a paired t-test of % As removal from jar tests that did use FeCl3 (p-value = 0.18; Table 4). That is, adding 50 mg/L of FeCl3 (17,000 μg/L of ferric iron, Fe3+) increased As removal from water without ambient Fe2+ (Table 4).
Wood ashes, calcic limestone (CaCO3), and dolomitic limestone (CaMg(CO3)2) are common and affordable in Bangladesh. Wood ashes are a byproduct from cooking fires, pottery kilns, and brick kilns. These limestones are used in agriculture to increase soil pH and provide nutrients for crop growth. In this study, wood ashes, calcic limestone, and dolomitic limestone were used to increase solution pH and promote the alkaline oxidation of soluble As(III) and soluble Fe(II) to insoluble As(V) and insoluble Fe(III). Oxidation with 1,000 mg/L of wood ashes followed by coagulation with 50 mg/L of FeCl3 was the only treatment that removed As to less than the 10 μg/L WHO drinking water guideline from water with and without ambient Fe2+ (Table 4).
It was originally thought that powdered brick might be an affordable oxidant and adsorbent for As removal. That is, Fe(III) from the brick might both oxidize As(III) and adsorb As(V). This mechanism, if present at all, provided negligible As removal (Table 4). However, 1,000 mg/L of powdered brick did on all occasions remove the yellow color caused by the oxidation of ambient Fe2+ and the use of FeCl3 as a coagulant (Table 4).
The control of the final pH must be improved (Table 4). The final pH must range from 5.5 to 8.5 (WHO 1984). Limestone was primarily used in these jar tests to promote the alkaline oxidation of As(III) and Fe(II) (Table 4). However, calcic limestone yields a highly buffered solution with a maximum pH of 8.2 and might best be used to adjust the final pH (Stumm and Morgan 1981).
Ambient Fe2+ did significantly increase As removal (p-value = 0.0003; Table 4). However, the distribution of elements in Bangladesh’s groundwater is highly variable and individual tubewells might not have enough Fe to coagulate As. In contrast, ambient Fe2+ did not significantly increase As removal if 50 mg/L of FeCl3 was added as a coagulant (p-value = 0.18; Table 4). Therefore, the need to use FeCl3 or a similar coagulant for affective As removal is a technical and economic problem.
In this study, wood ashes, calcic limestone, and dolomitic limestone were used to increase solution pH and promote the alkaline oxidation of soluble As(III) and soluble Fe(II) to insoluble As(V) and insoluble Fe(III). Oxidation with 1,000 mg/L of wood ashes followed by coagulation with 50 mg/L of FeCl3 was the only treatment that removed As to less than the 10 μg/L WHO drinking water guideline from water with and without ambient Fe2+ (Table 4). Therefore, the need to use wood ashes or a similar oxidizing agent for affective As removal is another technical and economic problem.
Unfortunately, the oxidation of ambient Fe2+ by wood ashes with the use of FeCl3 as a coagulant made the water yellow and the pH unacceptably high (pH = 9.8 ± 0.0; Table 4). Therefore, the need to use powdered brick for decolorization, and the need for calcic limestone or some other material for pH control are other technical and economic problems.
In summary, home-scale coagulation for removing As from western Bangladesh’s drinking water is most likely too problematic for effective use. First, soluble As(III) must be oxidized to insoluble As(V). Second, insoluble As(V) must be coagulated. Third, the water likely needed to be decolorized. Fourth, the pH must be controlled. Fifth, the inputs must be safe, simple, and affordable. Sixth, the sludge must be properly disposed. Moreover, effective treatment to remove the other toxic elements will likely cause even more problems.