This Standard Operating Procedure (SOP) was adapted from Frisbie SH, Mitchell EJ, Yusuf AZ, Siddiq MY, Sanchez RE, Ortega R, Maynard DM, Sarkar B. The development and use of an innovative laboratory method for measuring arsenic in drinking water from western Bangladesh. Environmental Health Perspectives 113(9):1196-1204 (2005).
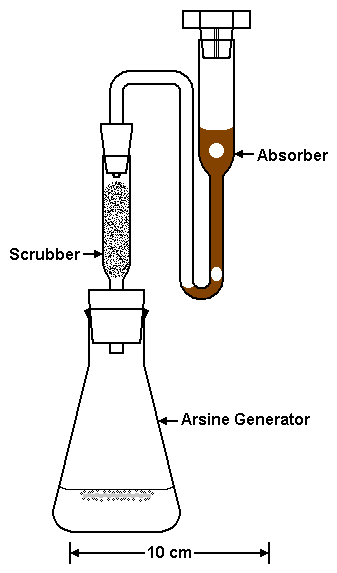 |
| Figure 1. The Arsine Generator, Scrubber, and Absorber. (Reproduced from Frisbie SH, Mitchell EJ, Yusuf AZ, Siddiq MY, Sanchez RE, Ortega R, Maynard DM, Sarkar B. The development and use of an innovative laboratory method for measuring arsenic in drinking water from western Bangladesh. Environmental Health Perspectives 113(9):1196-1204 (2005).) |
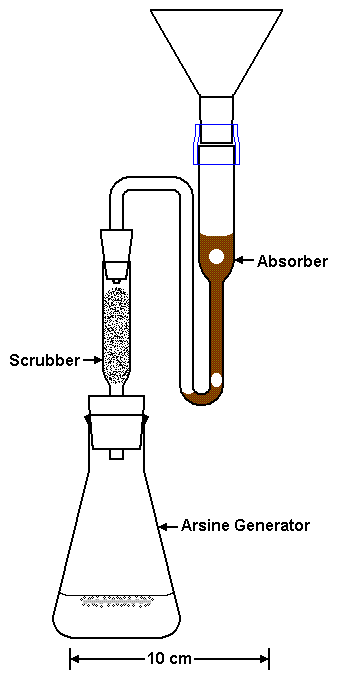 |
 |
| Figure 2. The Arsine Generator, Scrubber, and improved Absorber. | |
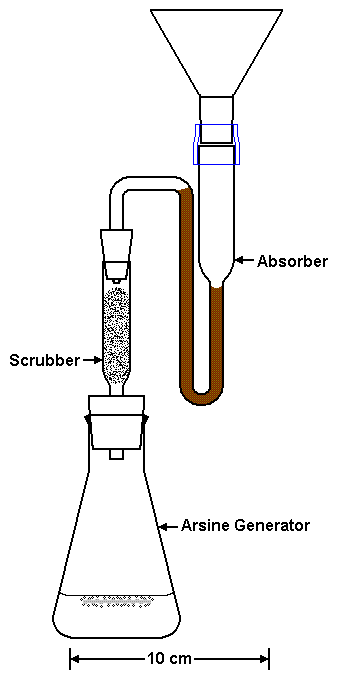 |
 |
| Figure 3. Do NOT let absorbate get sucked beyond this part of the Absorber. | |