The slope of Equation 1 and the 7-µg/L method detection limit shown in Table 1 suggest the colorimetric measurement of As by arsenomolybdate is extremely sensitive. The 101.5±3.6% recovery of known additions shown in Table 1 suggests the results from the arsenomolybdate method are accurate. The respective 2.1, 4.7, and 7.1 µg/L precisions of standards, samples, and known additions shown in Table 1 suggest the results from the arsenomolybdate method are reproducible.
| Arsenomolybdate | AgSCSN(CH2CH3)2 | GFAAS | |
| Method Detection Limit Equipment Cost Recovery of Known Additions c Precision of Standards Precision of Samples Precision of Known Additions Solvent |
7 µg/L $6,700 a 101.5±3.6% 2.1 µg/L 4.7 µg/L 7.1 µg/L H2O |
9 µg/L $6,700 a 103±18% 2.6 µg/L 4.5 µg/L 24 µg/L CHCl3 or C5H5N |
0.7 µg/L $37,000 b 103.3±3.1% 0.22 µg/L 1.7 µg/L 2.0 µg/L H2O |
a Includes a spectrophotometer, distillation unit for purifying laboratory water, analytical balance, top-loading balance, hot plate with stirrer, and glassware.
b Includes an atomic absorption spectrometer, distillation unit for purifying laboratory water, analytical balance, top-loading balance, and glassware.
c 95% confidence interval.
In this method, As is removed from the sample by reduction to AsH3 gas. This gas is collected in an absorber by oxidation to H3AsO4. All previous attempts at this separation have suffered from poor reproducibility due to the incomplete recovery of AsH3 (Sandell 1942; Sandell 1959). The sources of this incomplete recovery were insufficient concentrations of oxidant in the absorbate, the deterioration of absorbate with time, and improper absorber designs. This problem of incomplete recovery has been solved.
An error that has not been reported by previous researchers was discovered and corrected. This error was from drift caused by the arsenomolybdate absorption spectrum changing with time. Our method requires that the arsenomolybdate color develop for 30 min before its absorbance is measured. This color is relatively stable after 30 min. In addition, a loss of sensitivity from using polychromatic light to measure absorbance has been corrected. Our method requires that the absorbance be measured at 835 nm, the absorption maximum of arsenomolybdate. In addition, previous arsenomolybdate methods were not compared to established methods for measuring As (Milton and Duffield 1942; Sandell 1959). Our method is compared to the AgSCSN(CH2CH3)2 and GFAAS methods for measuring As (Tables 1-4).
Finally, all routine analytical methods must use a rigorous quality control plan to identify and correct systematic errors. The quality control plan for the arsenomolybdate method should include daily calibrations and the frequent analysis of blanks, externally supplied standards, calibration check standards, duplicate samples, and known additions of standard to samples (APHA et al. 1995; Frisbie et al. 1999; USAID 1997).
| Calculated t Critical 2-Tailed t at α = 0.05 with 70 Degrees of Freedom P-Value of Paired t-Test Correlation Coefficient, r Mean As Concentration by Arsenomolybdate Mean As Concentration by AgSCSN(CH2CH3)2 Relative Percent Difference of these Means, RPD |
1.88 1.99 0.06 0.993 27.6 µg/L 23.4 µg/L 16.6% |
However, the precisions of known additions for the arsenomolybdate and AgSCSN(CH2CH3)2 methods are different at the 95% confidence level (Table 1). These 2 precisions were measured using the same 8 samples. This suggests the AgSCSN(CH2CH3)2 method is imprecise at relatively high As concentrations due to the sample matrix. Additional evidence of this imprecision is the 16.6% relative percent difference (RPD) of the mean As concentrations from all 71 samples measured by the arsenomolybdate and AgSCSN(CH2CH3)2 methods (Table 2). One source of this imprecision may be the incomplete reduction of As(V) to As(III) in Bangladesh’s tubewell water by the relatively mild sample treatment procedure of the AgSCSN(CH2CH3)2 method. More specifically, the arsenomolybdate method uses KI and SnCl2.2H2O at 100º C for this reduction. In contrast, the AgSCSN(CH2CH3)2 method uses KI and SnCl2.2H2O at room temperature for this reduction. Another source of this imprecision may be the incomplete generation of AsH3 in Bangladesh’s tubewell water by the relatively mild AsH3 generation procedure of the AgSCSN(CH2CH3)2 method. More specifically, the arsenomolybdate method uses 0.18 g of KI, 0.540 g of SnCl2.2H2O, 2.0 mL of concentrated H2SO4, 10.0 mL of concentrated HCl, and 5.0 g of Zn per 35.0 mL of sample for AsH3 generation. In contrast, the AgSCSN(CH2CH3)2 method uses 0.300 g of KI, 0.16 g of SnCl2.2H2O, 5.0 mL of concentrated HCl, and 3.0 g of Zn per 35.0 mL of sample for AsH3 generation.
Another important difference between the arsenomolybdate and AgSCSN(CH2CH3)2 methods is related to worker health and environmental protection. More specifically, the arsenomolybdate method uses H2O as a solvent, which is nontoxic, nonflammable, and easy to dispose of. In contrast, the AgSCSN(CH2CH3)2 method uses either chloroform (CHCl3) or C5H5N as a solvent (Table 1). All routes of exposure to CHCl3 are likely to cause cancer in humans (U.S. EPA 2004). Moreover, CHCl3 can persist in an aquifer for centuries if it is improperly disposed of (Pankow and Cherry 1996). Exposure to C5H5N may cause increased liver weight and hepatic lesions (U.S. EPA 2004). In addition, C5H5N is highly flammable and as a result presents an acute risk to laboratory workers (Sittig 1985).
In summary, the arsenomolybdate method is more accurate and precise than the AgSCSN(CH2CH3)2 method based on the recoveries of known additions (Tables 1, 2). The arsenomolybdate method is safer than the AgSCSN(CH2CH3)2 method because it does not use toxic solvents (Table 1).
| Calculated t Critical 2-Tailed t at α = 0.05 with 70 Degrees of Freedom P-Value of Paired t-Test Correlation Coefficient, r Mean As Concentration by Arsenomolybdate Mean As Concentration by GFAAS Relative Percent Difference of these Means, RPD |
1.19 1.99 0.24 0.996 27.6 µg/L 28.6 µg/L 3.6% |
Despite its far lower cost, the arsenomolybdate method is more accurate than the GFAAS method. The recovery of known additions by the arsenomolybdate method is equivalent to 100% (101.5±3.6%; Table 1). In contrast, the recovery of known additions by the GFAAS method is greater than 100% (103.3±3.1%; Table 1). This suggests the GFAAS method overestimated the true concentration of As in this matrix by approximately 3.3% (Table 1). This estimate of 3.3% bias by the GFAAS method is based on the recovery of known additions from 8 samples. Additional evidence of this bias is the 3.6% relative percent difference of the mean As concentrations from all 71 samples measured by the arsenomolybdate and GFAAS methods (Table 3). This overestimation by the GFAAS method was likely caused by scattered light from sodium chloride (NaCl) or similar matrix salts that remained in the furnace during atomization (Frisbie et al. 1999; Frisbie et al. 2002; Harris 1999; USAID 1997). If so, the NH4NO3, Pd(NO3)2, and Mg(NO3)2.6H2O matrix modifiers and D2 background correction did not fully resolve this interference.
In contrast, the GFAAS method is more precise than the arsenomolybdate method. The standards, samples, and known additions are measured with greater precision by the GFAAS method than by the arsenomolybdate method (Table 1). Each of these 3 F-tests was evaluated at the 95% confidence level.
In summary, the arsenomolybdate method is more accurate and affordable than the GFAAS method (Tables 1, 3).
| Calculated t Critical 2-Tailed t at α = 0.05 with 70 Degrees of Freedom P-Value of Paired t-Test Correlation Coefficient, r Mean As Concentration by AgSCSN(CH2CH3)2 Mean As Concentration by GFAAS Relative Percent Difference of these Means, RPD |
2.63 1.99 0.01 0.995 23.4 µg/L 28.6 µg/L 20.1% |
Water testing and sharing can provide access to drinking water with As concentrations less than or equal to the 10-µg/L WHO guideline for the millions of Bangladeshis that live in communities where some tubewells are safe and others are not (Frisbie et al. 1999; Frisbie et al. 2002; USAID 1997). Whether or not this testing and sharing will actually provide safe drinking water in these communities will depend on neighbors correctly understanding the As results for their tubewells and consistently sharing safe water with each other. However, water gathering and usage are subject to cultural habits that are difficult to change. The nearly universal switch (97%) in Bangladesh since 1971 from using surface water for drinking to tubewell water has shown that water usage traditions can be changed, provided there are extensive community education programs and support from both the government and non-governmental organizations (WHO 2000).
In Bangladesh, as in most other areas where water must be gathered from a communal source, the chore of water gathering is generally considered to be women’s work. However, Bangladeshi women are constrained by society and their families to stay close to home, while unrelated men are generally not welcome inside family compounds. In order for water testing and sharing to be successful as a strategy for providing safe drinking water in Bangladesh, the neighbors as a community must be educated about the meaning of their As results. They must also be willing to use tubewells that may not be close to home, or to share their own safe tubewells with unrelated neighbors or strangers.
In our initial survey, which was completed before the owner or regular user of each tubewell knew the results from our testing, we asked respondents if they would be willing to use other water sources if their own tubewell had unsafe levels of As. We also asked them if they would be willing to share water with their neighbors if their own tubewell turned out to be safe. The results were very encouraging; 86% of respondents claimed they would permit family members to gather water from other tubewells if their own tubewell had unsafe levels of As, and 94% said they would share water with neighbors if they had safe water and their neighbors did not have safe water.
However, what people say they will do is not always what they actually do in practice. For this reason, we conducted a follow-up survey 6 months after our testing results were distributed to the original respondents or their family members. In this second survey, we learned that almost all tubewell owners (91%) shared water with others. Many respondents noted that sharing water with strangers is customary in village communities and required as a matter of courtesy or charity, which seems to override issues of water safety. Generally, however, following As testing, the status of a tubewell becomes known in the community. Once As test results become known in the community, only strangers continue to ask for water from contaminated tubewells. Owners of safe tubewells report that the largest group of non-family members gathering water at their tubewells are neighbors, and 26% of these owners claimed that 1 reason why they shared their water was because other tubewells had unsafe levels of As and theirs did not.
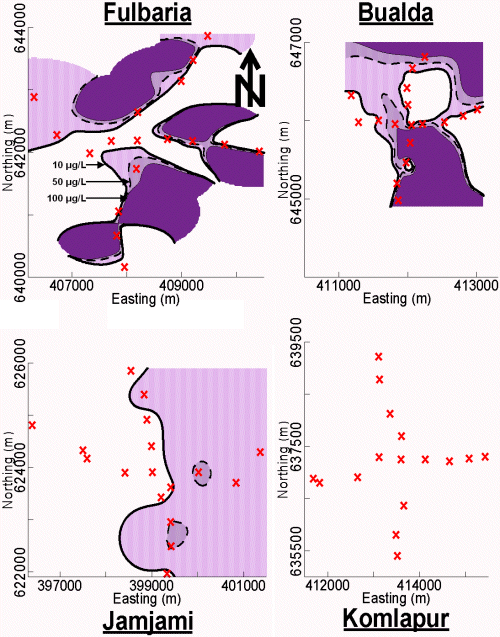
Instead of sending women out to get water from a stranger’s tubewell, men in some families took over this chore. Some men go to public tubewells at mosques to gather water for their families. Others seek water from neighbors' or relatives' tubewells. However, this was cited as a source of conflict from 11% of tubewell owners, who did not like having unrelated men enter their family compounds. The location of a tubewell is crucial. If the tubewell is located on the street, then all may use it freely. In contrast, if the tubewell is located inside a family compound, only family members and certain relatives have free access to the compound and the tubewell, regardless of the stated willingness of the owner to share the tubewell. Distance is also a factor for willingness to gather water from an outside tubewell, with one family reporting that it continued to use As-contaminated water because other tubewells were too far away. The maximum distance required to get safe drinking water in each of these 4 neighborhoods was approximately 0 meters in Komlapur, 490 meters in Bualda, 1400 meters in Fulbaria, and 2100 meters in Jamjami (see Figure 3).
 |
Education programs concerning the dangers of As-contaminated water have achieved some measure of success, since only one owner of an unsafe tubewell in our study appeared not to have understood the implications of his tubewell’s As results. This owner, a 70-year-old male, professed during the follow-up interview that his water was safe, and he told us that he had continued to use it and share it freely with others.
Many of the tubewell owners (85%) followed our recommendation and had their tubewells retested. This retesting was not done by our research team. Only one respondent told us that he thought retesting was not necessary (the one who believed his water was safe when it was not); the others who did not retest their water gave cost as the reason for not retesting. People were especially willing to retest their water if this could be done at no cost to them, with 96% of the respondents who had their water retested reporting that the retesting was done with no charge to them; however, 2 respondents paid to have their water retested.
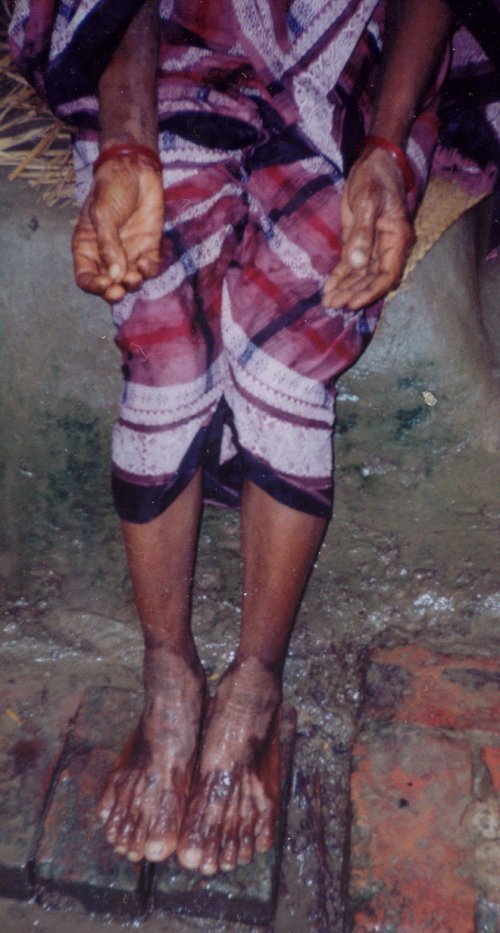
Our survey suggests the number of tubewells, especially private tubewells, is rapidly increasing in Bangladesh as more families acquire the economic resources to build them. While only 14% of tubewells were reported to have been constructed before 1983 (56% private), 27% were reported to have been constructed from 1983 to 1992 (83% private), and 59% from 1993 to 2002 (92% private), making the tubewells in our random sample from western Bangladesh only 9-years old on average. In addition, we asked whether respondents were aware of any As patients in the area. The number of nearby As patients was not statistically related to the concentration of As during our sampling event (p-value = 0.44). Similarly, the concentration of As during our sampling event was not statistically related to the age of the tubewell (p-value = 0.51). In contrast, the number of nearby As patients was statistically related to the age of the tubewell and hence the duration of exposure to tubewell water that was potentially contaminated with As. That is, the oldest tubewells were associated with the highest number of nearby As patients (p-value = 0.03). This stresses the fact that duration of exposure to As must be considered in addition to the concentration of As at any given time, since this can explain why the tubewells of some As patients are found to contain very low levels of As but have been used over many years (WHO 2001). For example, Figure 5 shows a female As patient with keratosis of the palms and blackfoot disease. When tested in 2002, the tubewell used by this patient had only 1.4 µg/L of As, but she had been drinking from this tubewell for 34 years. The As concentration in some of Bangladesh’s tubewells has changed dramatically over time (Frisbie et al. 1999; USAID 1997). These results demonstrate the need to periodically test all drinking water tubewells so that the total lifetime exposure to As can be reduced. Since most of the tubewells in the country have been installed quite recently, the numbers of As patients may begin to increase dramatically as more people develop a history of using tubewells for longer than the 5 to 10 years it may take to develop symptoms of chronic As poisoning. Finally, as the ages of the tubewells and the length of exposure to As increase, it may become even more vital to adopt a drinking water standard for As lower than the current Bangladesh standard of 50 µg/L, such as the 10-µg/L WHO guideline.
 |